Elevate Proliferation, Viability, and Therapy Potential
Reduce variability concerns with TheraPEAK® T-VIVO® Cell Culture Medium and achieve greater cell proliferation and viability, along with increased process efficiencies. Its novel chemically defined formulation is serum-free and non-animal origin (NAO), ensuring consistency and increased process control. Unlike many other serum-free media, this new medium does not need the addition of human serum (HS) to achieve the desired performance.
TheraPEAK® T-VIVO® Medium enhances the culture and expansion of:
With traceability documentation available, including Certificates of Analysis and a Drug Master File, TheraPEAK® T-VIVO® Cell Culture Medium simplifies processes, supports regulatory compliance, and scale up from preclinical development through to manufacturing.
TheraPEAK® T-VIVO® Medium enhances the culture and expansion of:
- αβ and γδ T cells
- CAR-T cells
- Peripheral blood lymphocytes (PBL)
- Tumor infiltrating lymphocytes (TIL)
With traceability documentation available, including Certificates of Analysis and a Drug Master File, TheraPEAK® T-VIVO® Cell Culture Medium simplifies processes, supports regulatory compliance, and scale up from preclinical development through to manufacturing.
TheraPEAK® T-VIVO® Cell Culture Medium is available as 1 L bottle or 1 L bag. Customization of packaging for specific applications is available.
Features and benefits at a glance
- NAO formulation; contains only recombinant proteins
- Does not require serum or serum component addition
- Greater cell proliferation
- Easy-to-use formulation only requiring addition of cytokines
- High transduction efficiencies and post-transduction viabilities
- Produced according to current GMP guidelines
Optimal performance across different platforms and T cell types
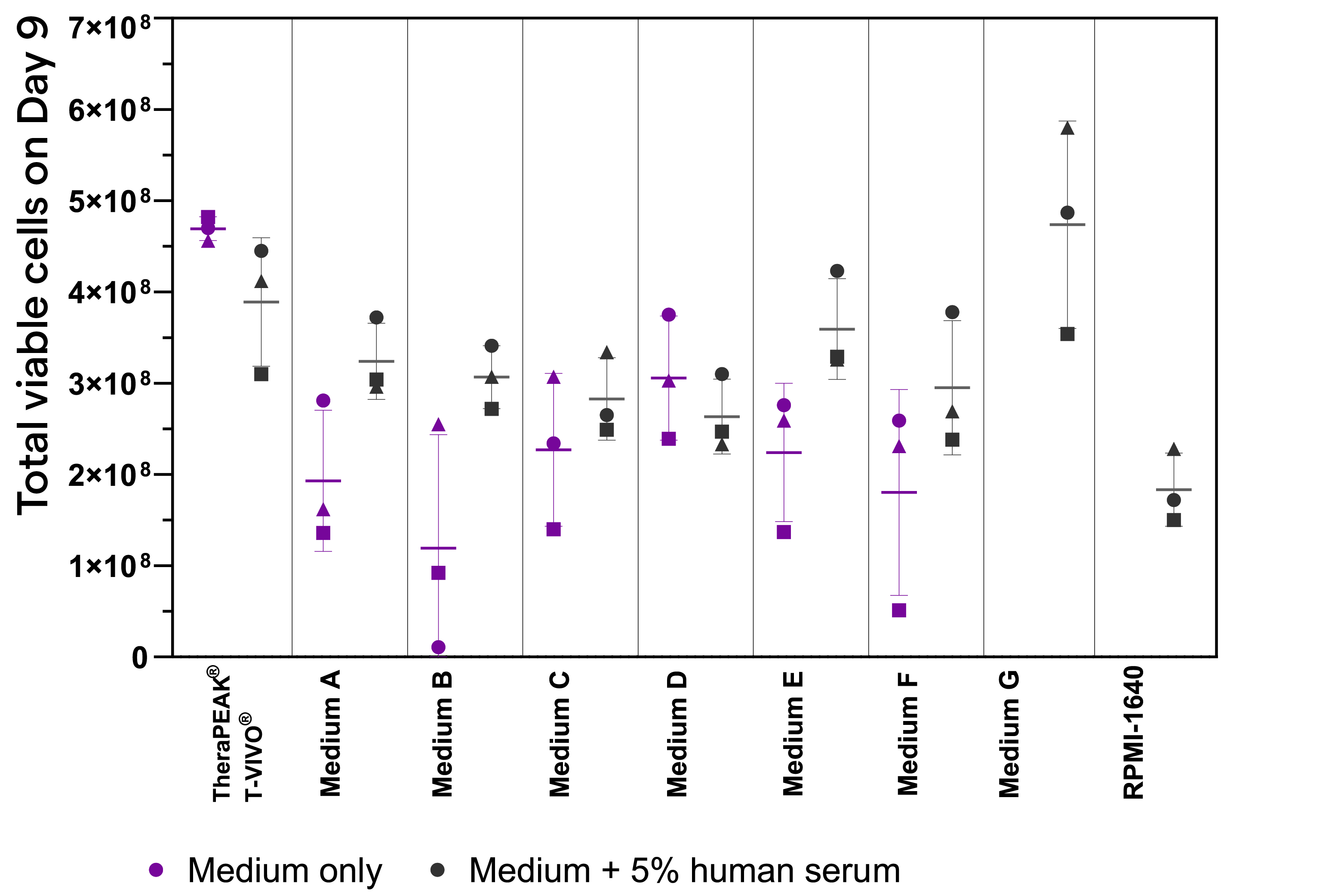
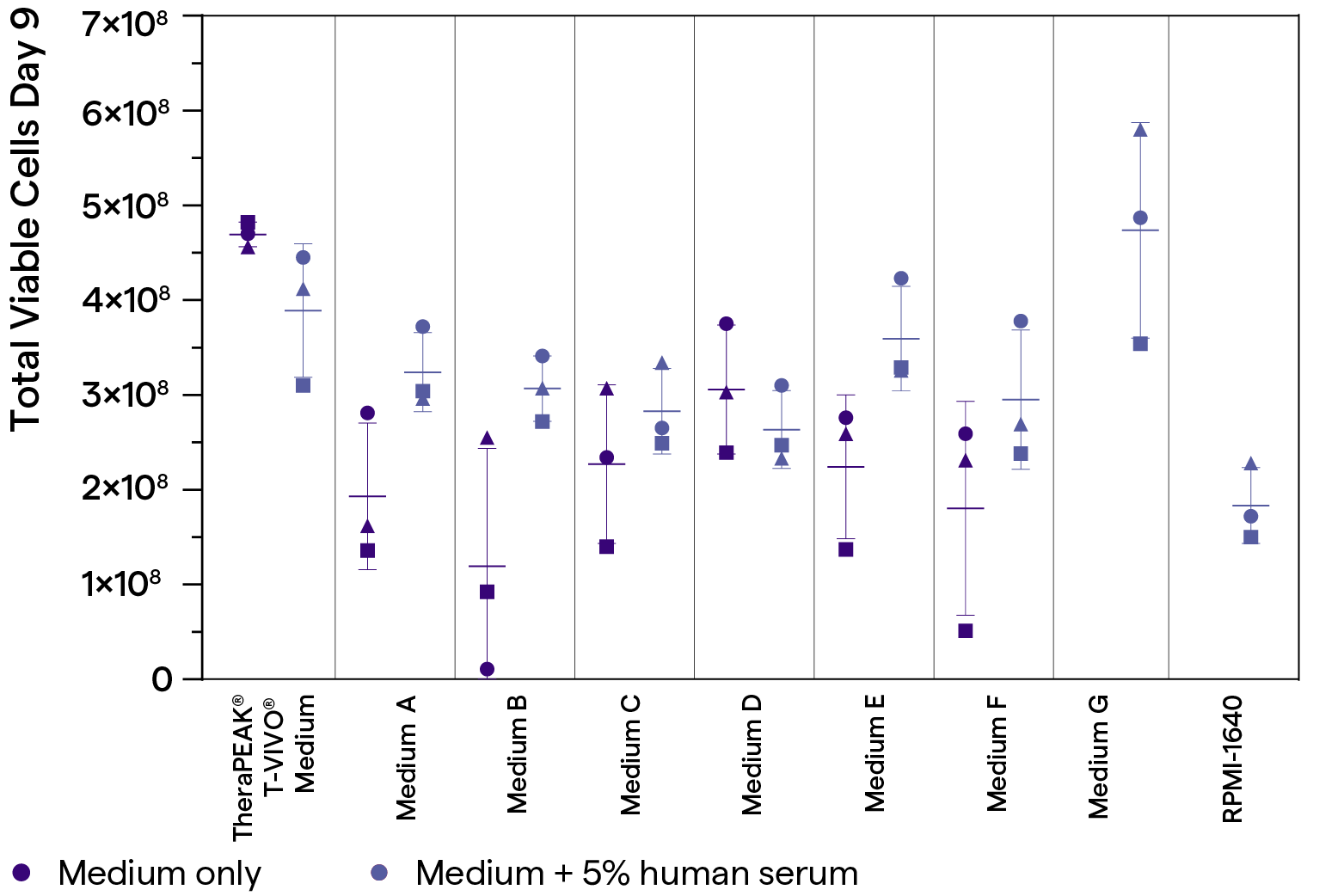
T-cell expansion in TheraPEAK® T-VIVO® Medium compared with other commercial media in a gas-permeable cell expansion device
TheraPEAK® T-VIVO® Cell Culture Medium supports efficient T-cell expansion in a gas-permeable cell expansion device compared with various commercial media. All culture media are supplemented with recombinant human IL-2 (100 IU/mL). Each donor is represented by the ▲■● symbol. Human AB serum (5%) is also added to each medium for comparison purpose (black symbols). Medium G and RPMI-1640 do not support T cell growth without the supplementation of human AB serum.
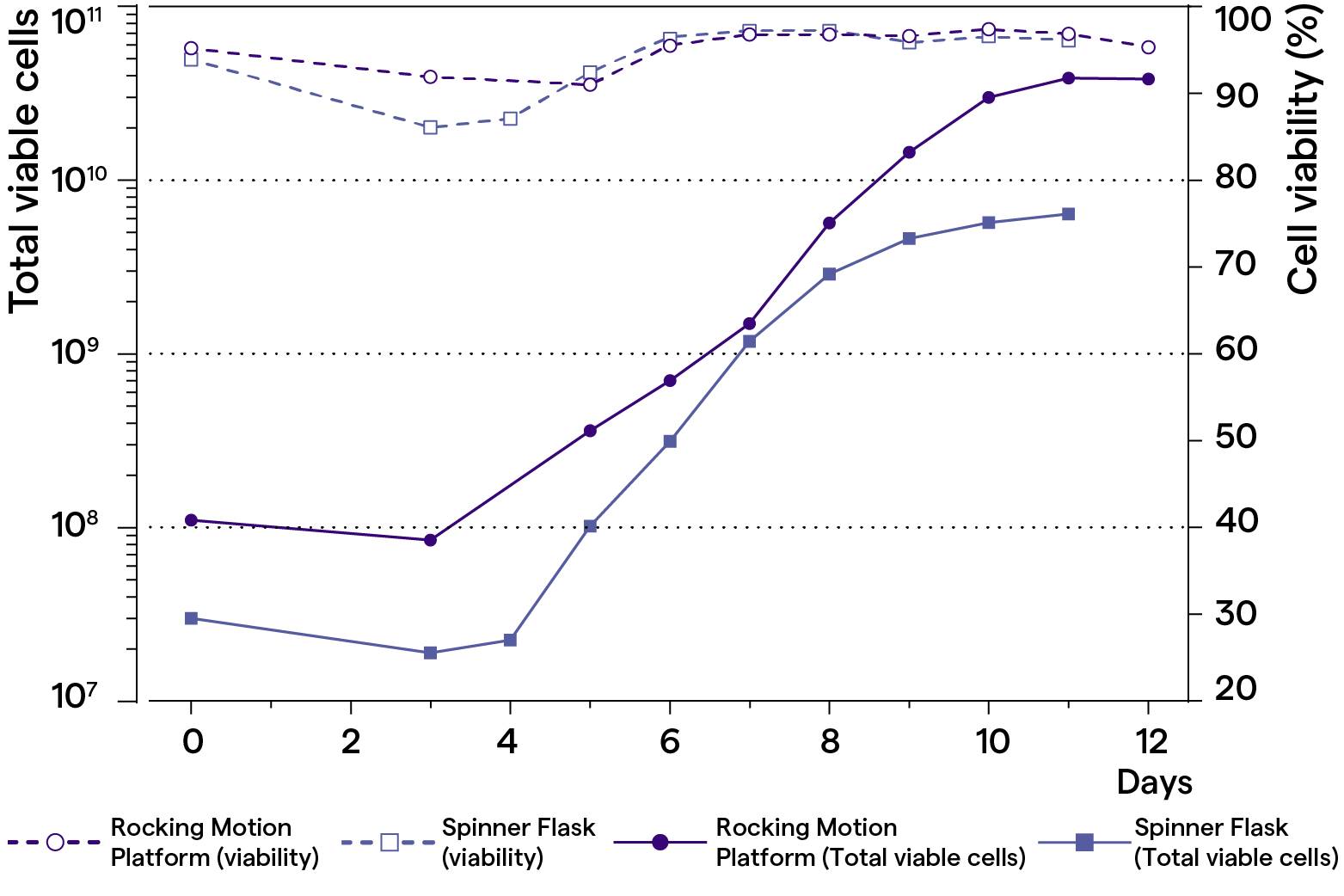
Scale-up of T-cell expansion in rocking motion platform and spinner flask using TheraPEAK® T-VIVO® Medium
Full-scale T-cell expansion in spinner flask or rocking motion platform bioreactor. Peripheral blood mononuclear cells are activated via CD3 and CD28 and expanded with TheraPEAK® T-VIVO® Medium for up to 12 days. TheraPEAK® T-VIVO® Medium is supplemented with recombinant human IL-2 (100 IU/mL).
Would you like to see more data? View our scientific poster
- A Novel, Chemically Defined Medium Supports Superior Cross-plattform T-cell Expansion
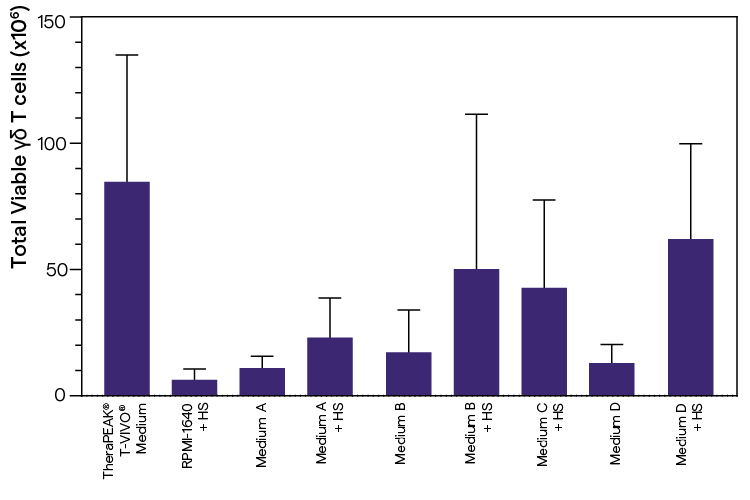
γδ T-cell expansion from PBMCs in T-flask
γδ T-cell expansion out of PBMCs (2.0 x 106 cells). Recombininant human IL-2 (200 IU/mL) is used in all media.
Would you like to see more data? View our scientific poster
- Two Distinct Activation Methods Yield Clinical-Scale Expansion of Peripheral Blood Derived γδ T Cells